Diagnostics on Cancer Tissue
In the USA there are approximately 1.7 million new patients diagnosed each year with late stage cancer. Drug treatment for most of these patients is standard non-selective chemotherapy with all its well-known complications, but newer approaches involve two groups of drugs targeting either specific DNA cancer mutations (targeted chemotherapy) and/or enhancers of the immune system (check point inhibitors for specific immunotherapy).
However, due to the inadequate sensitivity of currently available molecular diagnostic assays for the target mutations, use of these new drugs is currently confined to late stage cancers (Stage IV).
The much higher sensitivity of MutantDx assays will enable these drugs to be used for earlier stages with anticipated enhanced treatment outcomes.
Targeted Chemotherapy
The optimal standard of care now requires that the cancer be sampled and tested for the specific DNA mutations that might qualify a patient for targeted chemotherapy. Failure to test for and detect these specific driver mutations would result in standard chemotherapy and compromised survival. The various cancer mutations may be a changed DNA sequence (e.g. BRAFp V600E/K), or a deleted base pair (e.g. EGFR del L747-A750).
Testing for such mutations is now being extended to earlier stages of cancer (<Stage IIIa), substantially increasing the market size for these newer drugs.
At an early stage, a cancer is smaller and has lower cancer burden compared to late stages, and there is therefore the need for much more sensitive tests for detection, especially as the sample is increasingly obtained via a fine needle biopsy resulting in very few cancer cells being aspirated.
MutantDx uses its patented Allele Specific Multiplex Sequencing (ASMS) technology to detect DNA mutations in cancer samples at a concentration 1,000 times lower than presently used methods, offering substantially more patients the benefits of targeted chemotherapy.
Applications of MutantDx technologies have demonstrated the ability to detect and verify the following mutations in cancer tissue samples:
- Braf p. (V600E/K)
- EGFR (L858R, Del 747A-750, T790M)
- Kras (G12V, G12D, G13D)
Check Point Inhibitors
These drugs work by inhibiting a cancer cell’s ability to mimic a normal cell surface and thereby evade detection by the immune system, which would otherwise identify the cancer cell as ‘not self’ and destroy it. MutantDx’s patented RepSeqID and SeqVar technologies can detect gene deletions (splice variants) and gene fusions (e.g. Alk and ROS) for the selection of patients who are likely to respond to specific immunotherapy. No competitor offers such triaging capability.
Examples of Check Point Inhibitors are PD-1 and PD-L1.
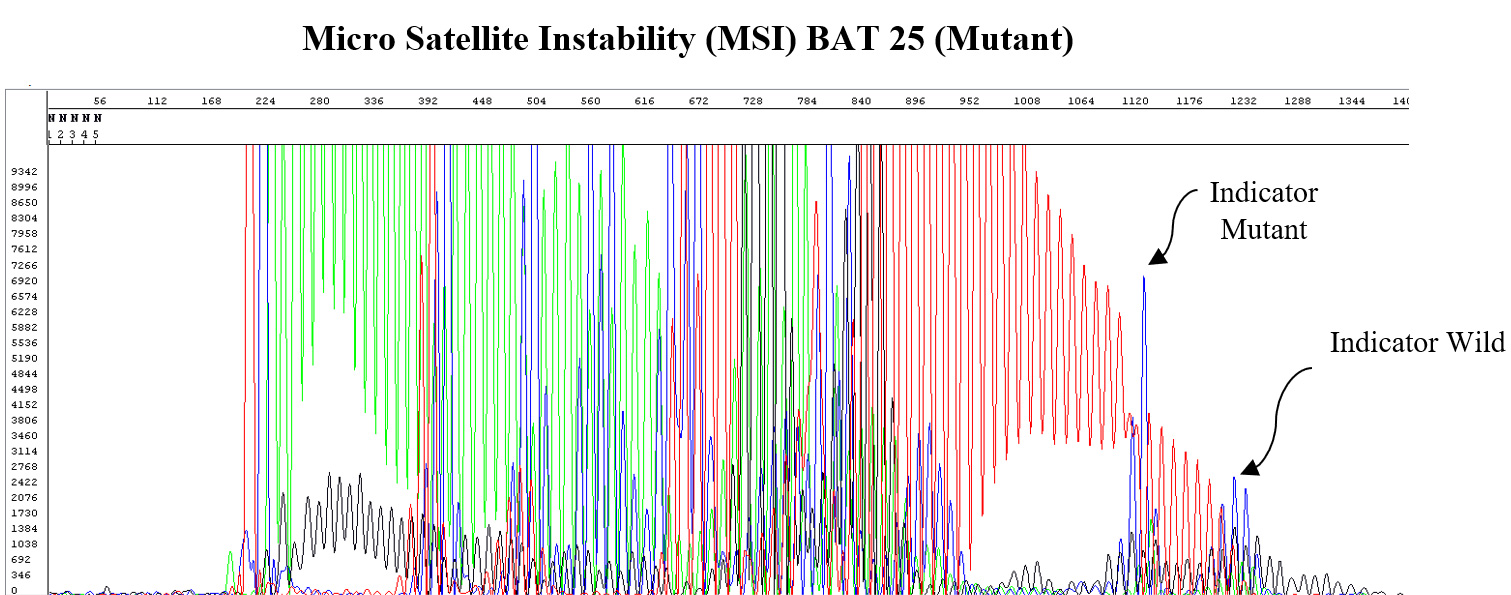
Micro-Satellite Instability (MSI)
MSI refers to the degree to which an individual tumor shows a defective DNA repair mechanism as manifested by short repeats of the same nucleotide (e.g. MSI-H and MMR). This gives a measure of the tumor’s prognosis and susceptibility to certain drugs.
MutantDx uses its RepSeqID platform to assess MSI.
Dr Roger Hodkinson
MutantDx Inc.
Edmonton, Alberta
Canada T5S 1E6