Market Potential
Market Potential of the MutantDx Liquid Biopsy
The Liquid Biopsy has the potential to address three distinct actionable objectives in clinical oncology:
The identification of specific DNA mutations that allow the selection of targeted chemotherapy for newly diagnosed cancer patients
Monitoring the treatment of cancer patients for the potential of early recurrence due to clonal variations (additional DNA mutations conferring resistance to the first line therapy). That ability allows new treatment modalities to be rapidly devised.
Regular screening of individuals, triaged for risk factors, to identify the very earliest stage of an asymptomatic cancer
The rate of adoption of the Liquid Biopsy for these transforming objectives will be based on the already rapidly accelerating development of new drugs for actionable mutations, the sensitivity/specificity of competing technologies, cost/reimbursement issues, and proven clinical utility. The use of the Liquid biopsy is still in its infancy but is projected to grow exponentially, and hence the market potential is outlined in two categories of Present and Future:
1. Present market potential
-
Monitoring first treatment outcomes for specific markers associated with targeted chemotherapy
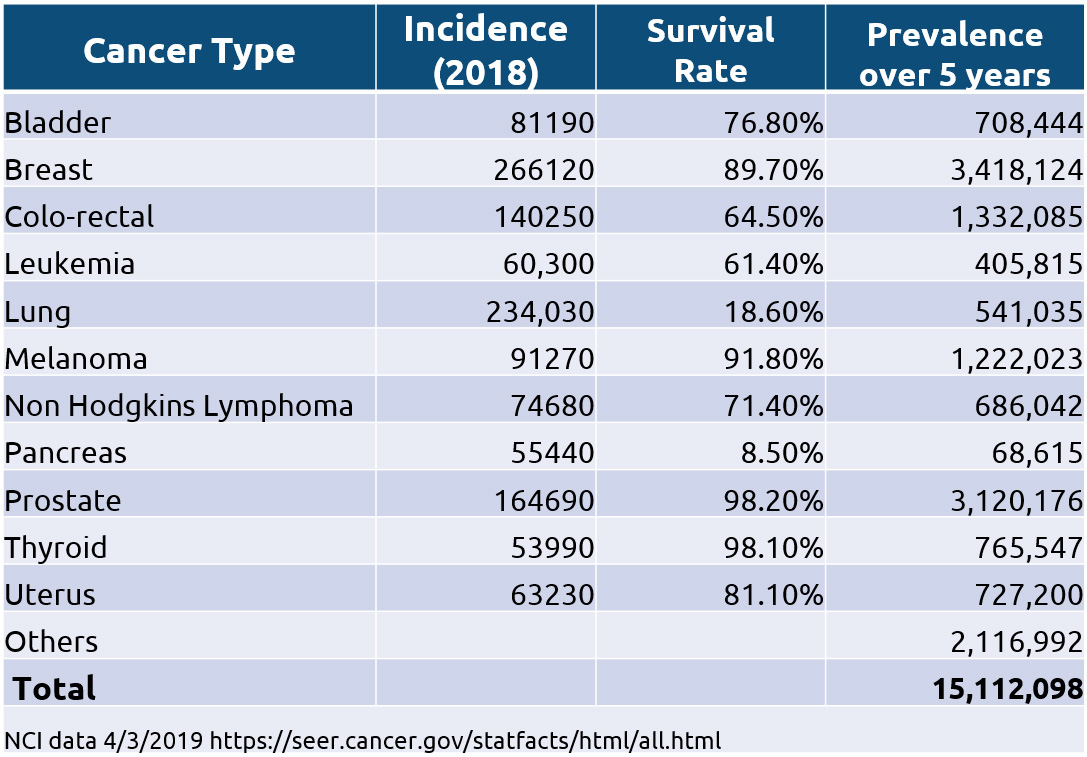
There are more than 1.7 million new cancer patients annually in the USA, for some of whom targeted chemotherapies are available (lung, colorectal, melanoma, and prostate cancers). However, the two malignancies investigated the most often with the liquid biopsy are lung cancer and melanoma. There are 234,030 new lung cancer patients and 91,970 melanoma patients annually in the USA. This is currently reimbursed by Medicare at $175 (CPT-81210) for Braf p.600E/K, and $350 (CPT-81235) for EGFR L858R, T790M, del 747_A750. The total current market potential is estimated at $ 97M per annum.
- Monitoring for cancer recurrence due to clonal variations
Potential revenue from this group will be the same as the above, with the only difference being that these patients are tested periodically over an extended period of five years. The total market potential is estimated at $ 97M per annum. - Screening high risk patients
The only high volume screening for cancer at this time is for colorectal cancer. In 2015 there were 15 million colonoscopies. Medicare reimbursement for a multi-mutation Liquid Biopsy panel for colorectal cancer would be $492.72 resulting in a total market potential of $ 7.4B per annum.
2. Future market potential
A large number of research initiatives are currently underway to discover cancer specific mutations for which specific chemotherapy drugs will be developed. Given the current pace of discovery, over the next 5-10 years it is likely that most types of cancer will have identified markers that direct treatment. The future market potential is estimated as follows:
-
Monitoring first treatment outcomes for specific markers associated with targeted chemotherapy
It is estimated that there will soon be over 2 million new cancer patients annually in the USA, and most of them will have available targeted chemotherapies for corresponding cancer mutations. At an average $400 reimbursement per sample, the total market potential is ~$ 800M per annum.
- Monitoring for cancer recurrence due to clonal variations
Assuming prevalence of all cancer in the USA is 15 million patients, at an average $400 reimbursement per sample the total market potential is $ 6B per annum. - Screening the asymptomatic population
Once cancer type specific markers are established there will be regular screening of the asymptomatic population. This will probably consist mainly of seniors who constitute 16% of the US population, representing 56 million people. At an average $400 per sample the total market potential is $ 22B per annum
Dr Roger Hodkinson
MutantDx Inc.
Edmonton, Alberta
Canada T5S 1E6