Verifiable Specificity
Verifiable Specificity = Virtual Elimination of False Positives
The clinical consequences of false positive results are disastrous:
- treating a condition that is not there, with all the potential side effects
- unnecessary anxiety for the patient, and
- unnecessary cost
There are only two general methods for mutation identification:
A labeled probe
A probe is a short synthetic DNA molecule designed to be complimentary to a mutated DNA target in the Liquid Biopsy, and onto which it is expected to bind – analogous to a ‘Lock and Key’ process. This is a non-enzymatic process with end point of a non-verifiable light signal. The uncertainty of the probe binding to the correct DNA target, and the non-verifiability of the result, can increase the false positive rate with this technology. While lower concentrations of the mutation target can result in false negative results.
DNA sequencing
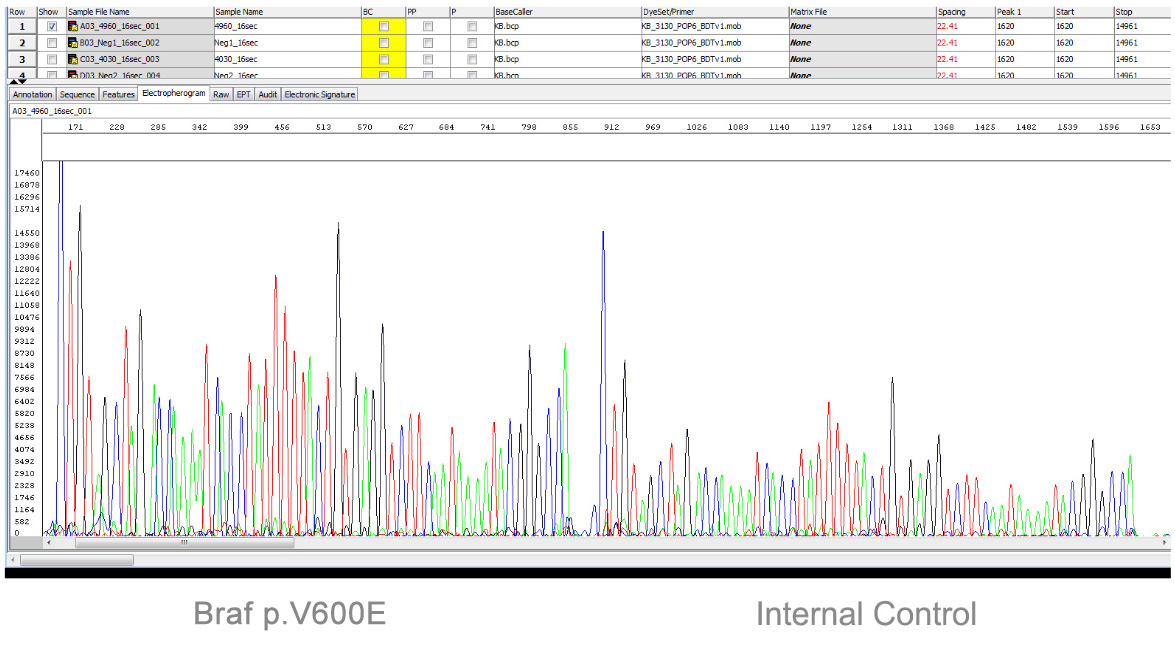
DNA sequencing is an enzyme mediated process that has the ability to generate correct nucleotide sequences and verify positive results. Of the three sequencing platforms currently in clinical use (Sanger, Pyrosequencing and NGS), only Sanger sequencing is accepted as the Gold Standard method. MutantDx’s PrimaCap patent is a novel modification of Sanger sequencing, providing absolute verification of a positive result.
Reference 1, 2 and 6
Typical Mutant Dx Sequencing Result
Dr Roger Hodkinson
MutantDx Inc.
Edmonton, Alberta
Canada T5S 1E6