Unique QA Patents
Detection of mutations from clinical samples is subject to false positive and false negative results. MutantDx virtually eliminates these problems using patented QA methods unavailable to our competitors, creating greater confidence in patient results.
False Negatives
This is usually caused by a failure to amplify or identify the target DNA mutation(s).
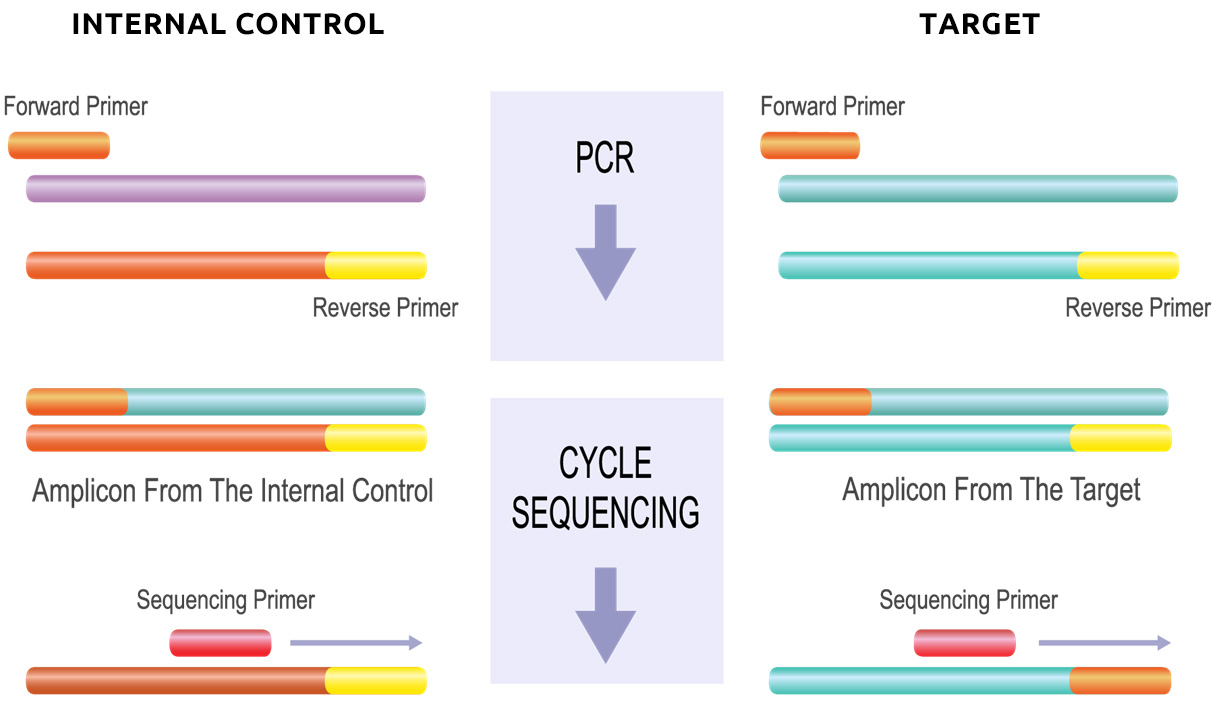
Only MutantDx’s patented platform technologies provide an internal test control where the reagents (primers) used for amplification and identification of DNA in the patient’s Liquid Biopsy are the same as those used for the internal control in each test panel.
References 5, 6 and 8
False Positives
This is usually caused by cross-contamination between samples when tested as a batch on automated instrumentation and/or the labeled probe binding to the wrong target. MutantDx allows this possibility to be immediately recognized by intentionally seeding each patient sample with a unique short DNA molecule (an oligonucleotide).
Reference 2, 6, 8 and 10
Technical Details
Dr Roger Hodkinson
MutantDx Inc.
Edmonton, Alberta
Canada T5S 1E6